Polyurethanes
Properties
Polyurethanes (PUR) are one of the largest classes of polymers with properties that can be tailored over a wide range for a large number of application. They can be thermosetting or thermoplastic, rigid and hard or flexible and soft. They are formed from the reaction of an organic diisocyanate with a diol compound, which leads to urethane linkages in the backbone (-NH-C(=O)-O-).
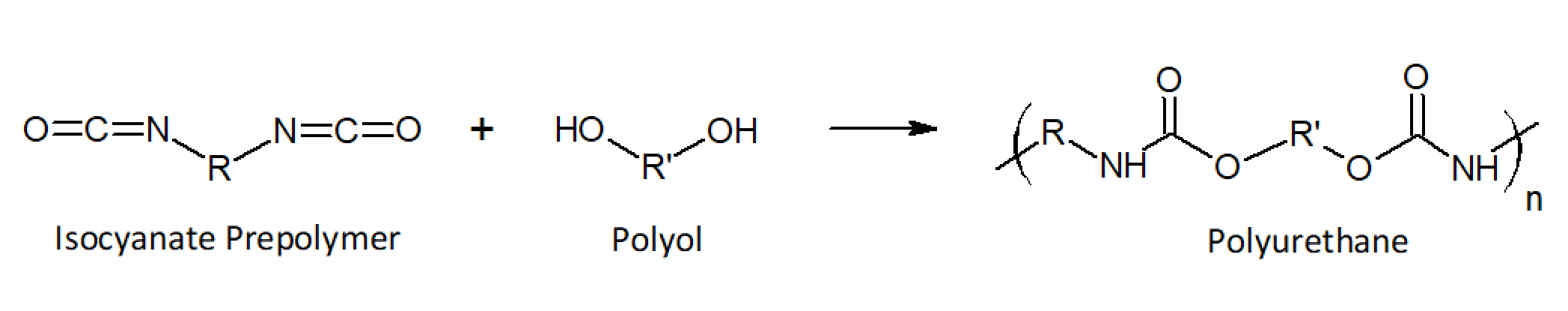
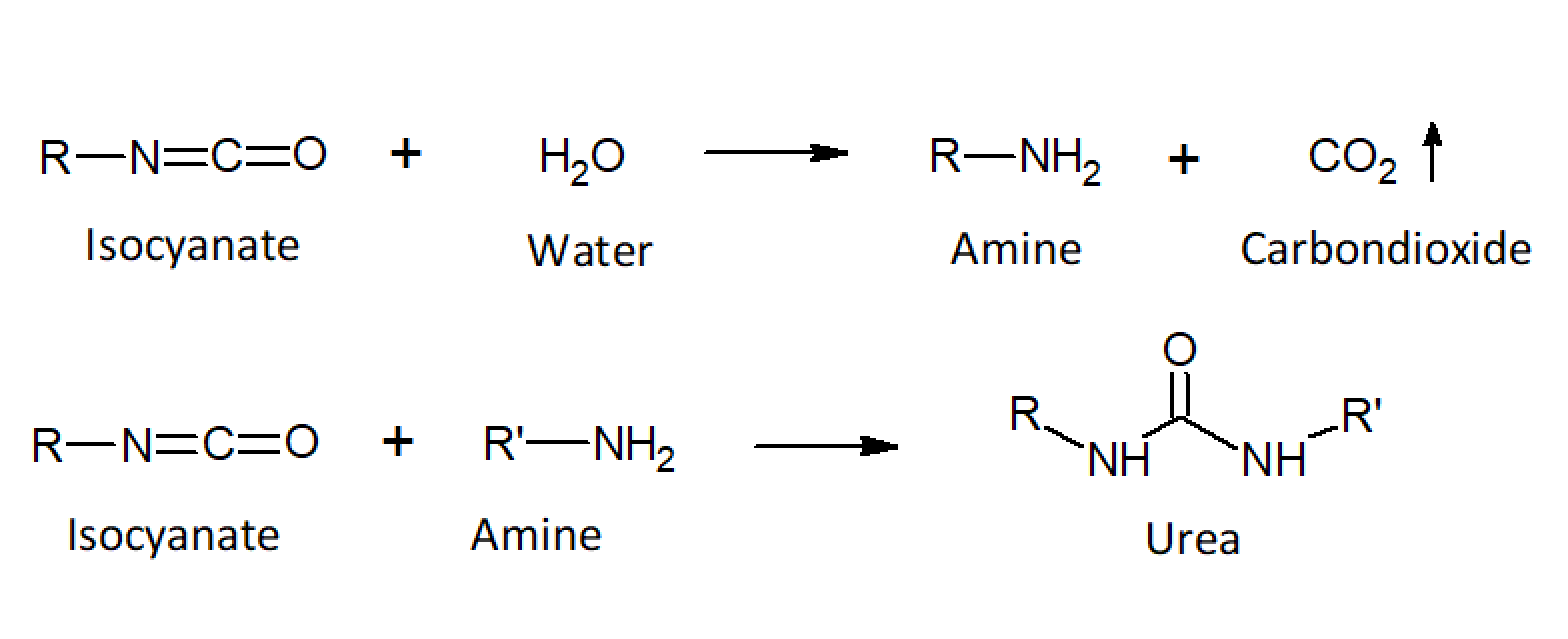
The high reactivity of the isocyantes allows for reaction injection molding and for the manufacture of rapid curing adhesives and coatings. The manufacture process often involves two steps. First, a low-molecular-weight polyol is reacted with an excess isocyanate to form an isocyante prepolymer. These prepolymers are than coupled (vulcanized) with a diol compound during molding. Foamed PUR structures are produced by deliberately adding a small amount of water. The isocyanate groups react with the water to urea linkages (-NH-C(=O)-NH-), which releases carbon dioxide that acts as a blowing agent during molding.
The properties of PURs greatly depend on the structure of the polymer backbone. They can be tailored to have high strength, high rigidity or high flexibiltiy and toughness. Most of these urethanes have good resistance to oil, (aromatic) hydrocarbons, oxygen, and ozone. Two major drawbacks of PURs are their susceptibilty to microbial attack and the tendency of aromatic urethanes to discolor (yellow) when exposed to UV light.
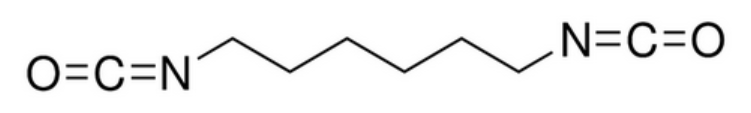
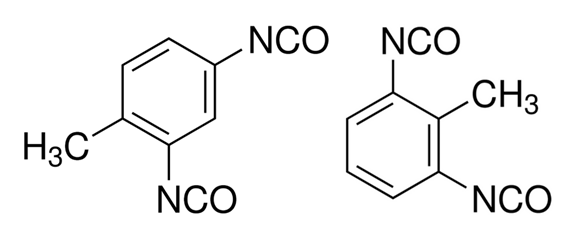
The three most important commercial aromatic isocyanates are toluenediisocyanate (TDI), diphenylmethane diisocyanate (MDI), and naphthalene diisocyanate (NDI) and their polymeric forms. Toluene diisocyanate is usually supplied as a mixture of two isomers: 2,4-TDI and 2,6-TDI (see table below). MDI and TDI are often used for the preparation of thermoplastic elastomers and foams, while the polymeric forms of MDI and TDI are often prefered for coatings, sealants and adhesives. Aliphatic isocyanate of industrial importance are hexamethylene diisocyanate (HDI), isophorone diisocyanate (IPDI) and hydrogenated MDI (HMDI). In contrast to aromatic isocyantes, urethanes made with these isocyantes are UV-stable (do not discolor) and are less susceptible to oxidation and degradation. However, they are more expensive. For this reason, aromatic isocyantes are used when oxidative discoloration on exposure to UV radiation is not an issue, whereas aliphatic isocyantes are preferred for more demanding applications, like exterior coatings. An important triisocyanate is triphenylmethane triisocyanate. It finds applications in the coating and adhesive industries.
Polyurethanes obtained from aromatic isocyantes and low molecular weight diols have high glass transition temperatures and are rather brittle, whereas PURs obtained from aliphatic isocyantes and polymeric diols have much lower glass transition temperatures (Tg's) and are much more flexible. The majority of commercial PURs are elastomers. They can be made without crosslinking (vulcanizing) the polymer chains. The highly elastic network consists of alternating flexible polyether/polyester blocks and rather short and hard (aromatic) urethane blocks. The urethane blocks associate to hard domains that are connected via hydrogen bonds. These domains act as physical crosslinks and provide a restorative force when the thermoplastic urethane elastomer is stretched.
Most elatomeric polyurethanes are either polyester or polyether based.
The soft segments comprise the larger portion of the elastomer and, therefore, determine the
physical properties of the elastomer. For example, polyester-based urethane elastomers have better
oxidative and high temperature stability than polyether-based polyurethanes, but have lower
hydrolytic stability and low-temperature flexibility.
However, polyethers are usually more expensive than polyesters.
The cheapest polyether
polyol is hydroxyl-terminated polypropylene oxide (PPO), also called polypropylene glycol
(PPG).
Another important
polyether polyol is based on polytetramethylene oxide (PTMO), sometimes called polytetrahydrofurane
(PTHF). Both PTHF and PPO polyols have low melting temperatures and very low Tg's (190 - 200 K).1
Polyurethanes based on PTHF typically have higher strength than PPG
based urethanes, probably due to PTHF's ability to crystallize under stress.
It is the preferred polyol for the manufacture of elastic urethane fibers such as Spandex (Elastan) for stretchable fabrics.
Many polyester polyols are made from adipic acid and ethylene glycols (polyethylene adipate), or from
butanediols and adipic acid (polybutylene adipate). Both diols are crystalline above room temperature.
To reduce the Tg and to destroy the crystallinity, copolyesters are often prepared from a mixture
of glycols and adipic acid. Another important polyol is polycaprolactone diol. It is a biodegradable
polyester with a low melting point of about 330 K and a glass transition temperature of about 210 K.
It is sometimes copolymerized to reduce the crystallinity in the caprolactone based oligomer.
This diol is mostly used for the manufacture of speciality polyurethanes.
COMMERCIAL POLURETHANE PREPOLYMERS AND POLYOLS
Two of the largest producers of isocyantes and polyurethane (prepolymers) are Covestro (formerly Bayer) and BASF. Some important isocyantes together with their chemical structure and trade names can be found in the table below.
| Isocyanate | Structure of Repeat Unit | Manufacturer |
| Tolylene-2,4-diisocyanate (2,4-TDI) Tolylene-2,6-diisocyanate (2,6-TDI) |
| Bayer Mondur®, BASF Lupranat® |
| 1,4-Phenylene diisocyanate (MDI) |
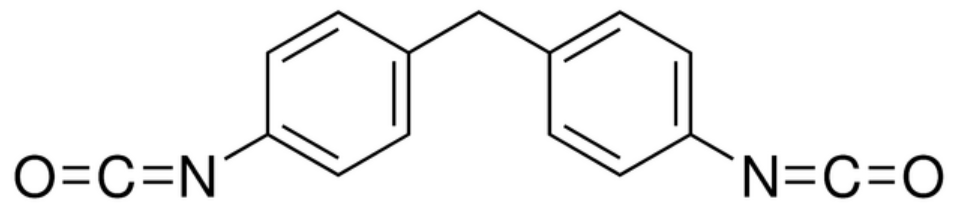
| Bayer Mondur®, BASF Lupranat® |
| Isophorone diisocyanate (IPDI) |
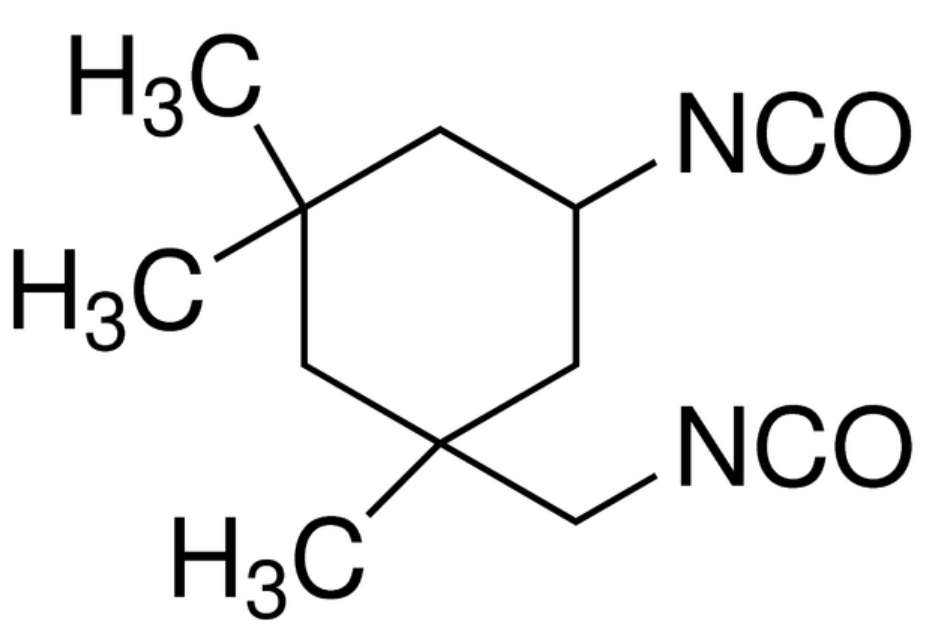
| Bayer Mondur® |
| Hexamethylene diisocyanate (HDI) |
| Bayer Mondur® |
Covestro and BASF are also major producers of polyols which they market under the trade names Desmophen®, Acclaim® (Covestro), and Lupranol®, Lupraphen®, Pluracol®, Poly THF® (BASF)
APPLICATIONS
The versatile urethane chemistry allows for the production of a wide range of products including
flexible and rigid foams, solid elastomers, extrusion and
injection-molded parts, coatings, sealants, and adhesives.
Flexible, high-resilience foamed products include mattresses, upholstered furniture, carpet underlays
and auto parts like cushions, backs, and armrests. Rigid foamed products with a closed cell morphology are used as
insulations for commercial and residential buildings. Other applications of
rigid foam include insulations for tanks, pipes, water heaters, refrigerators, and freezers.
Examples of solid elastomeric products are durable elastomeric wheels and tires for forklifts,
skateboards, roller coasters and escalators. Elastomeric urethanes find also many uses in the
automotive industry. Some examples are bumpers, fenders, steering wheels, instrument and door
panels, and gaskets for trunks, windows and windshields.
Polyurethanes are also used as electrical potting compounds, adhesives, coatings, sealants, and
for the fabrication of synthetic fibers (Spandex).
1Due to the low polarity and high flexibility, PPG and PTMO polyols are liquid at room temperature, whereas most higher molecular weight polyester polyols are crystalline greases.